Fundamental Science
Research Direction 1:
Reverse neurodevelopmental disorders
Restoring the expression levels of disease risk genes to reverse the progression of neurodevelopmental disorders
Our research strategy aims to identify common molecular-level abnormalities induced by various NDD risk gene mutations, and to develop pharmacological approaches that rectify the perturbations in the expression level of disease risk genes to relieve NDD symptoms regardless of the complexity or uncertainty of the causes. A major obstacle to developing effective therapy to manage brain disorders is the lack of drugs that could modulate the expression level of disease risk genes, such as KCC2 in which reduced gene expression has been associated with many brain disorders.
To bridge this severe disconnection between disease risk gene discovery and drug development, we have pioneered a target gene-specific high-throughput drug screening platform based on human stem cell-derived neurons that carry a luciferase reporter in the endogenous KCC2 gene. Screening of chemical drug libraries with such a platform led to the identification of the first group of small molecule compounds that enhance the expression of the KCC2 gene in neurons, which rescued disease-related deficits at the cellular and behavioral levels in cell and mouse models of Rett syndrome (Tang et al., Science Translational Medicine, 2019). Our unbiased screening results shed light on previously uncharacterized molecular pathways regulating KCC2 gene expression. The discovered small molecule compounds that activate KCC2 gene expression can potentially be applied to treat many brain disorders caused by E/I imbalance.
Leveraging the gene editing and stem cell research workflow, we will investigate small molecule and mRNA formulations that regulate expression levels of a number of disease risk genes to rescue disease phenotypes caused by gene haploinsufficiency. We will also investigate the subcellular localization and protein interaction partners of the protein products encoded by these risk genes.
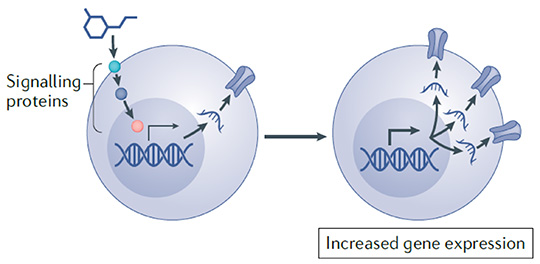
Develop novel therapeutics to modulate the expression levels of the genes that encode the target proteins. (Tang et al., Nature Reviews Neuroscience, 2021)
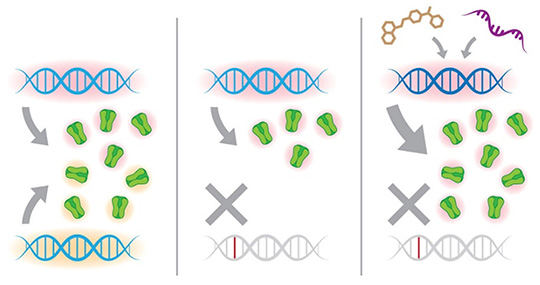
Enhance the transcriptional and translational output of the intact wild-type genetic allele to rescue risk gene haploinsufficiency. (Tang lab, 2021)
Publications
1. Tang, X., Drotar, J., Li, K., Clairmont, CD., Brumm, AS., Wu, H., Liu, XS., Wang, J., Gray, NS., Sur, M., Jaenisch, R.. Pharmacological Enhancement of KCC2 Gene Expression Exerts Therapeutic Benefits on Human Rett Syndrome Neurons and Mecp2 Mutant Mice, Science Translational Medicine, 2019. PMID: 31366578. (highlighted by F1000Prime and Nature Reviews Drug Discovery)
2. Li, C.H., Coffey, E.L., Dall’Agnese, A., Hannett, N.M., Henninger, Tang, X., J.E., Oksuz, O., Zamudio, A.V., Schuijers, J., Liu, X.S., Markoulaki, S., Svoboda, D.S., Wogram, E., Lee, T.I., Jaenisch, R., Young, R.A., Dual roles for MeCP2 in heterochromatin condensate formation and separation from transcriptional condensates, Nature, PMID: 32698189.


