Fundamental Science
Research Direction 3:
Organoid Models of Brain Tumor
Brain cancer, such as high-grade glioma and craniopharyngioma, are the most common forms of solid cancer in the U.S and the leading cause of cancer-related death among children and adolescents ages 0-19 years. Current research models of brain cancer mainly rely on genetically inducing tumor growth from mouse brain cells in vivo, transplanting ex vivo mouse or human tumor cells into the mouse brain, or growing human tumor cells in vitro. These models are insufficient for studying the complex genetic, cellular, and tissue underpinnings of the human brain tumor microenvironment. As a result, a large fraction of the candidate therapeutics validated using these models show poor clinical translatability for improving care practices for brain cancer patients.
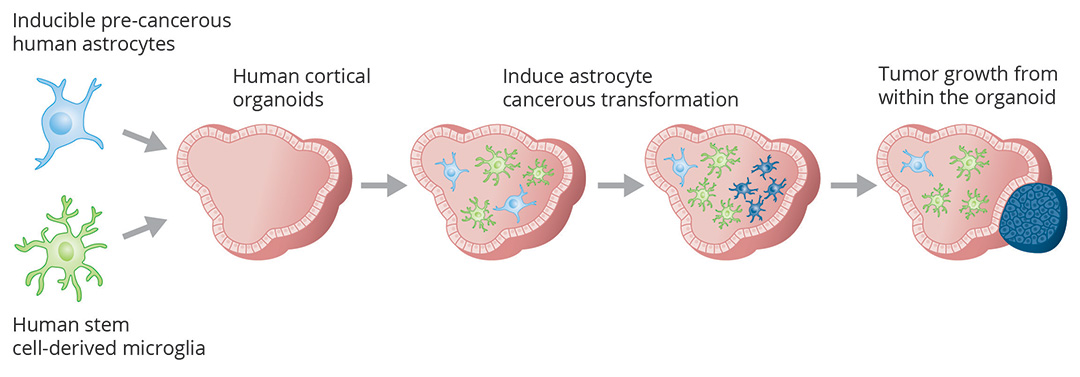
Integrating hESC-derived astrocyte and microglial cells into cortical organoids, and inducing cancerous transformation of astrocytes inside the brain organoids. (Tang lab, 2021)
Brain tumors closely interact with the surrounding tissue to promote their growth and to evade immune surveillance. For example, recent work has uncovered bi-directional interactions between neurons and tumors through electrochemical synapses and paracrine factors, and demonstrates that tumor-induced KCC2 expression reduction and resulting epilepsy in turn provides trophic support to the tumors and further promotes their growth. Therefore, tumor-induced KCC2 reduction results in a ‘vicious cycle’ that involves a positive feedback between brain hyperexcitability and tumor growth, which represents a potential target for therapeutic intervention.
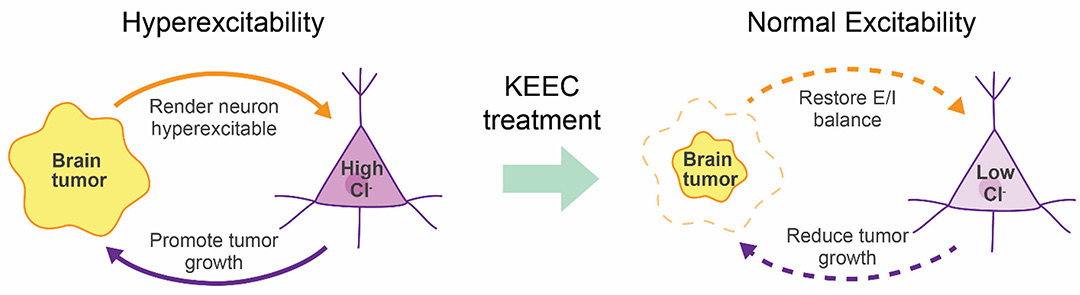
Suppress tumor growth through activating KCC2 gene expression to normalize neuronal excitability. (Tang lab, 2021)
We aim to leverage our expertise in gene and cell engineering to develop a new generation of 3D human brain tumor models containing the main cell types in the brain to faithfully recapitulate the tumor microenvironment. Such models will also be valuable for investigating the interactions between cancer and immune cells during brain tumor progression or elimination. In order to validate the organoid system in modeling brain tumor, results generated from the brain tumor organoids will be compared to those obtained from surgically-resected primary brain tumor tissue.
Our reproducible and scalable 3D human brain tumor organoid model will provide unprecedented ease of access to characterize the molecular and cellular events during tumor origination, progression, and elimination. A long-term goal is using our organoid model to discover quantitatively distinct and programmable cellular states that can be leveraged to devise therapeutic strategies to halt brain tumor progression or enhance the effectiveness of existing tumor therapeutics to clear residual cancer cells after brain tumor surgery.
Publications
1. Sarnow K., Majercak E., Qurbonov Q., Cruzeiro GAV., Jeong D., Haque IA, Baird LC., Filbin MG., Tang X., Neuroimmune-competent human brain organoid model of Diffuse Midline Glioma, Neuro Oncology, 2024, PMID: 39561098


