The Woolf Lab is driving scientific understanding of the complex neurobiology that underlies pain, regeneration of the injured nervous system, inflammation and neurodegenerative diseases. Our work is concentrated on primary sensory and motor neurons as well as the spinal cord and cortex, and on the interaction of neurons and immune cells, using a multidisciplinary approach spanning stem cell derived neurons, molecular and cell biology, electrophysiology, optogenetics, neuroanatomy, live cell imaging, behavior and genome editing.
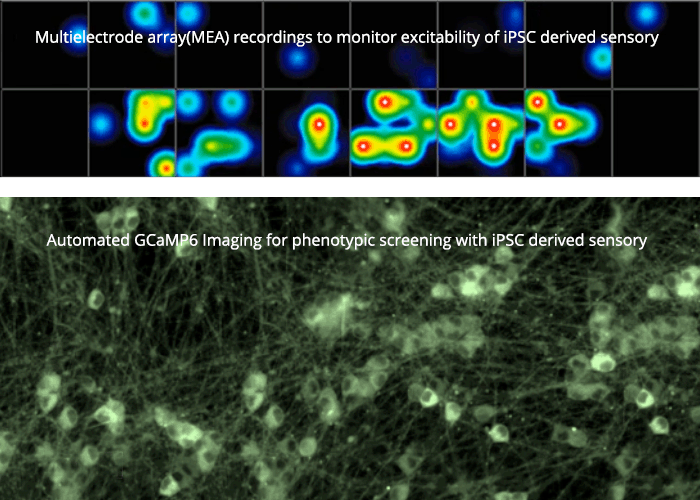
We have established functional and genomic strategies using single cell expression profiling, bioinformatics and gain- and loss-of-function approaches to screen for and validate novel genes that contribute to neuronal plasticity and diverse disease phenotypes. We also use human stem-cell-derived neurons to explore physiology and pathophysiology and for high content phenotypic screens.
We are formulating a new Translational Systems Biology of Pain, Regeneration and Neurodegeneration, and apply genomic and compound screens to identify potential therapeutic targets in pursuit of:
- innovative analgesics that do not confer the risk of abuse and addiction,
- novel neuroprotective treatments for conditions like ALS and peripheral neuropathy, and
- anti-inflammatories that target neuroimmune interactions.
Pain
We are looking for non-opioid pain medications and new therapies for nerve regeneration and degeneration. We use stem cell technology and CrispR gene editing to create new cell lines that mimic or correct patient conditions. Such human-derived sensory neurons enable us to model disease in a dish and to test novel compounds that block those proteins involved in the generation and persistence of pain, the amplification of inflammation and in promoting regeneration and halting degeneration.
(Image Credit: Jaehoon Shim, PhD)
This video was made by our proprietary “PalmReader” machine. The system tracks mouse behavior via imaging and supervised and unsupervised machine learning based computational analyses. We use such behavioral assays to study neurological diseases and assess the efficacy of potential therapeutics.
(Credit: Nivanthika Wimalasena)
Through “neurophotonics” we obtain a literal window on the neurons in the brain and spine of living model animals. Multiple neuron types are implicated in pain, and we are able to track them in real time.
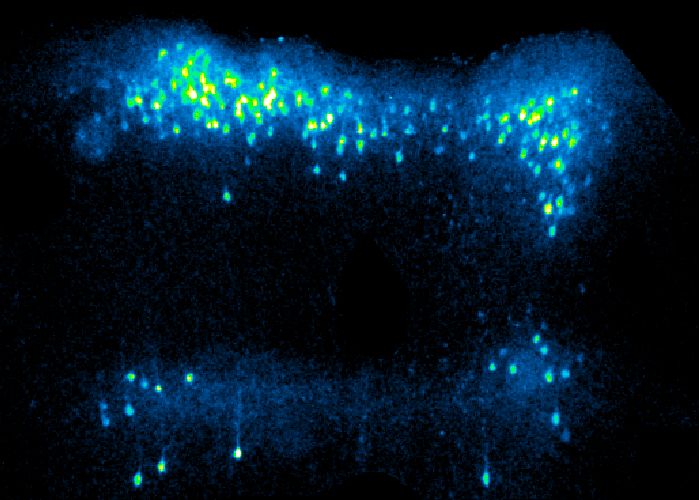
This photo shows cortical neurons lighting up in a live mouse as they process and interpret sensory and pain signals. We trace and record the signals as they pass between the brain and the periphery.
(Image by Daniel Taub, PhD)
Nerve Regeneration
Two videos and a photo demonstrate axons in an iPSC-derived human spot culture, cut through by a laser beam, and their subsequent regeneration after application of certain drugs. We use an automated imaging system that records and analyzes the activity in live cells. This enables us to observe changes in human neuronal spot cultures and to study “axonal injury in a dish.” We test and analyze the effects of compounds that may promote regeneration after injury.
(Credit: Szu-Yu (Tammy) Ho, PhD)

We use “Incucyte,” an automated imaging system that records and analyzes the activity in live cells. Incucyte enables us to observe changes in our spot cultures and to study “injury in a dish.” We test and analyze the effects of target compounds that may promote regeneration after injury.
Nerve Degeneration
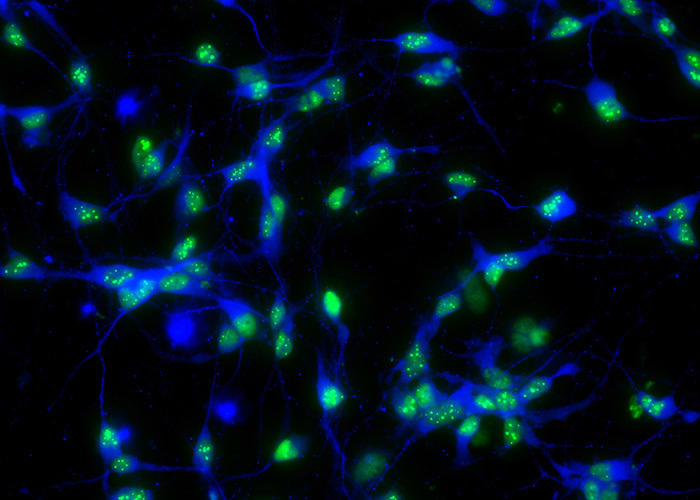
What causes neural circuits to break down in the event of neurodegenerative diseases? By studying motor and other neurons derived from patient stem cells, we trace the genetic pathways implicated in diseases like amyotrophic lateral sclerosis (ALS).
(Image “TDP-43 aggregation in human iPSC derived neuron” from project “exploiting stem cell technology to study regeneration and degeneration.” created by Kuchuan Chen, PhD.)
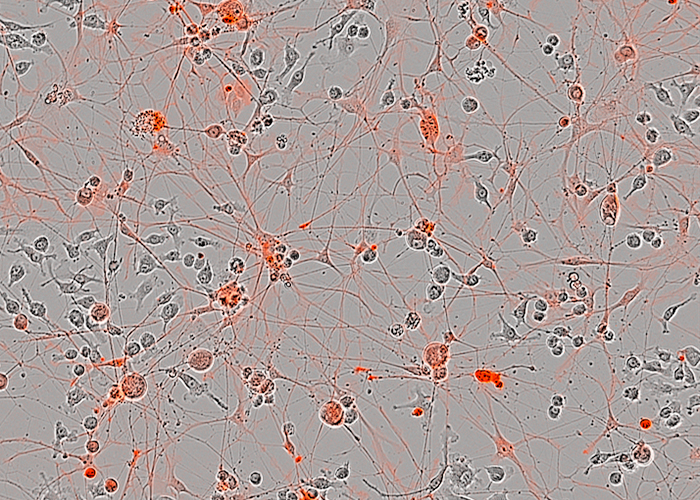
We also use stem cell and CrispR models to test novel therapies for chemotherapy-induced peripheral neuropathies. About 40% of patients receiving chemotherapy develop a neuropathy and currently there is no treatment for this.
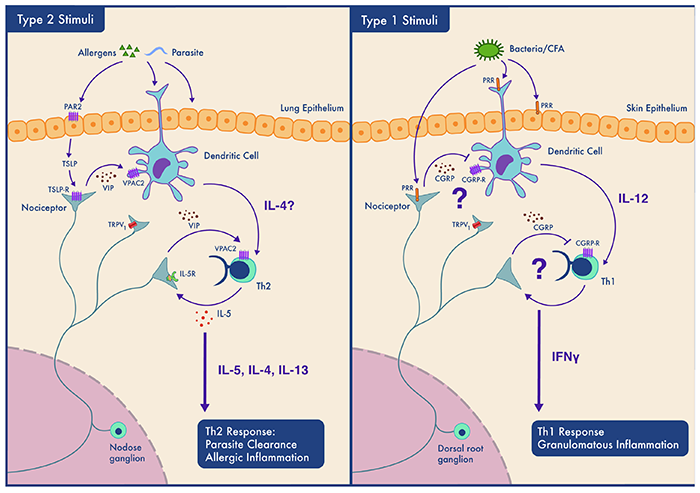
Neuro-Immune System Interactions
Certain neurons are specialized to communicate with the body’s immune cells. When the normal crosstalk among neurons and immune cells is disrupted or augmented, disease can occur. Diseases of the lung and skin (asthma, dermatitis, psoriasis) appear to originate from neuro-immune interactions. Transcriptomic and proteomic analyses allow us to scrutinize these interactions and draft a map of the neuro-immune interactome.
Novel small molecules screens enable us to identify proteins that silence those neurons that normally activate the immune system to block or reduce inflammation.
Our group works closely with many academic groups and the pharmaceutical industry, both to model disease and to identify molecular targets for novel analgesics, axonal growth determinants, anti-inflammatory and neuroprotective agents.
We have ongoing major collaborations with Bruce Bean and David Ginty (HMS Neurobiology), Kevin Eggan (Harvard Stem Cell and Regenerative Biology), Dan Geschwind (UCLA Neurology), Peter Sorger (Lab of Systems Pharmacology, HMS) and Stephanie Lacour (EPFL, Geneva).
The Neurobiology of Pain
Click on the image below to view a nine-part video (42 minutes total).
Dr. Clifford Woolf describes the mechanisms of normal pain sensation and the mechanisms of pain transmission that result in pathologic pain syndromes. He presents an approach to pain syndromes base on these mechanisms for chronic pain treatment.
Initial publication: October 02, 2015
Pain Podcast
What is the nature of physical pain? Why do we even experience it? Is there one type, or many? Do people experience pain differently? What is happening in our brains and our bodies when we experience pain? What is the biological link between pain and addiction? Dr Woolf is interviewed in a 37-minute podcast recorded September 17, 2019.