The Engle Lab
at the F.M. Kirby Neurobiology Center
Patient & Study Participant Resources
Welcome, we are glad you found us!
You may have found this web page because you, a family member, or your patient, have/has been diagnosed with a rare condition affecting eye and/or facial movements. If so, we hope that the following information will address questions you may have about enrolling in our research and/or attending our CCDD Consultation Clinic.
From a research standpoint, the Engle Lab strives to better understand the neurogenetic basis of cranial nerve development and function. This is done through gene discovery and basic research described in the Science section of our website. Gene discovery requires the enrollment and participation of individuals and families diagnosed with rare conditions involving atypical or limited eye and facial movements. These conditions include both defined or undefined congenital cranial dysinnervation disorders (CCDDs) and familial strabismus.
Dr. Engle and her colleagues also oversee and participate in a multi-disciplinary, second-opinion consultation clinic at Boston Children’s Hospital for children and adults diagnosed with ocular CCDDs. This clinic is staffed by two physicians, Dr. Elizabeth Engle, a child neurologist and pediatrician, and Dr. David Hunter, an ophthalmologist, as well as an orthoptist and genetic counselor. More information on this clinic is provided in “Attending the Ocular CCDD Clinic”.
- See our FAQs section for definitions and additional background.
How can I participate or become involved?
There is overlap between the conditions studied by the Engle Lab and the conditions seen through the CCDD clinic at Boston Children’s Hospital. However, the research and formal medical evaluations are performed through separate programs. People can choose to participate in, and be appropriate for, one or both options. To pre-screen individuals for either program (research enrollment or clinic visit), Dr. Engle and her team review clinical information provided through forms and medical records. This helps us to be sure that the desired study and/or clinic participation are appropriate.
Continue reading below to learn more or click on the buttons to move to a specific section.

Dr. Elizabeth Engle (center, front) and members of the Engle Lab commemorating Moebius Syndrome Awareness Day 2020.
Enrolling in Engle Lab Research Studies
The Engle Lab’s research enrollment goals are to (1) enroll participants who are born with complex eye and facial movement disorders or disorders of lower cranial nerve function together with family members and (2) enroll families in which three or more relatives are diagnosed with strabismus. Patients, parents, health care providers and other researchers all refer individuals to us for enrollment.
Examples of disorders we study:
- Complex congenital eye and eyelid movement disorders:
- Congenital Fibrosis of the Extraocular Muscles (CFEOM)
- Congenital ptosis
- Marcus Gunn Jaw Winking syndrome (MGJWS)
- Duane syndrome / Duane retraction syndrome (DRS)
- Horizontal Gaze Palsy (HGP)
- Horizontal Gaze Palsy with Progressive Scoliosis (HGPPS)
- Moebius syndrome / Moebius sequence (MBS)
- Congenital 3rd, 4th, or 6th nerve palsy
- Ocular and lid synkinesis
- Any other complex congenital eye or lid movement disorder (yet to be defined)
- Congenital facial weakness or palsy
- Isolated congenital facial weakness
- Syndromic congenital facial weakness
- Moebius syndrome
- Congenital disorders of lower cranial nerves
- Examples include disorders of tongue movement, chewing, or swallowing
- Familial Strabismus
- Families in which 3 or more relatives are diagnosed with strabismus, including but not limited to esotropia, infantile esotropia, accommodative esotropia, intermittent esotropia, and exotropia.
The conditions we study generally cause symptoms that are noted in infancy or early childhood, even if an official diagnosis is not made for several years. If symptoms begin in adulthood or are due to surgery or trauma, our study is unlikely to be appropriate. Please do not hesitate to contact us with questions about eligibility.
What participation includes:
- All participation steps can be arranged remotely. Travel to Boston is not required.
- There are no fees nor costs to participate.
- We can explain the study and enrollment process and answer any questions via telephone, video conferencing, or secure email. If a potential participant is being seen at Boston Children’s Hospital, we may also be able to meet in person.
- Enrollment is ongoing and rolling with no predetermined end date.
- Our goal is to enroll all affected and unaffected close members of a family (such as parents and siblings) when feasible.
- Enrollment consists of four steps: 1) Screening, 2) Consenting, 3) Request for medical records, imaging, and test results, and 4) Sampling.
1. Screening
Receiving initial information about potential participants through a screening form allows us to determine if enrollment in our study is appropriate. We gather this information through an Intake Form that can be downloaded or collected by phone. Once completed, please return the form via email to englegc.research@childrens.harvard.edu or by regular mail.
2. Consenting
Informed consent from each participant is obtained. Informed consent is a legal term. It means that the potential participant is fully aware of the facts and risks of participating in the research study before agreeing to it. An Engle Lab staff member will obtain consent by phone or videoconference. This session provides an opportunity for the potential participant and family to ask questions and for us to be sure that everyone is aware of the study process. For minors or people under guardianship, in addition to consent, we may obtain assent from the participant. For families with appointments at Boston Children’s Hospital, in-person consenting may also be an option. Depending on the discussion and number of questions, the consenting process typically takes 10-20 minutes. Participants are given an opportunity to read the consent carefully prior to the discussion, and forms can be emailed directly and/or included in an enrollment kit. Once all questions have been satisfactorily addressed and consenting has been completed and is on file, we move on to sampling.
3. Request for medical records, imaging, and test results
In addition to the initial intake form, we require additional clinical information to successfully study an enrolled individual or family. This information can be obtained throughout the enrollment and participation process. We request that the participants fill out the forms below: clinical history form, medical history form, and medical records release form that provide permission for us to request medical records and copies of imaging studies (typically MRI scans). Please note that some facilities require that patients use that facility’s specific release form; information on our form should help you to complete any requests to share records with us. We also ask for photos and videos of eye and facial movements (some of which can be done online). Please download these forms by clicking below. Once completed please return them to us via email to englegc.research@childrens.harvard.edu or by regular mail. Links to additional forms including a sample consent are also available in the FAQs below.
What forms or information are required?
4. Sampling
The Engle Lab genetic studies typically require a saliva or blood sample from each participant. Once received by the lab, this sample is used to extract DNA. Saliva samples can be obtained in the comfort of the home, using special collection tubes that we can mail, along with some paperwork, directly to you or to your doctor’s office. These samples and paperwork are then returned to the lab using our mailers at our cost. As with the other steps, if you are receiving care at Boston Children’s Hospital, we can arrange to meet with you in person to complete sampling. On rare occasions when the lab cannot successfully obtain adequate DNA from a sample, we may need to request a repeat saliva sample or an alternative sample, such as via a blood draw. On other rare occasions a participant may prefer to provide a blood sample rather than a saliva sample.
Please do not hesitate to contact us with any questions about the enrollment process.

Dr. Elizabeth Engle and Dr. David Hunter.
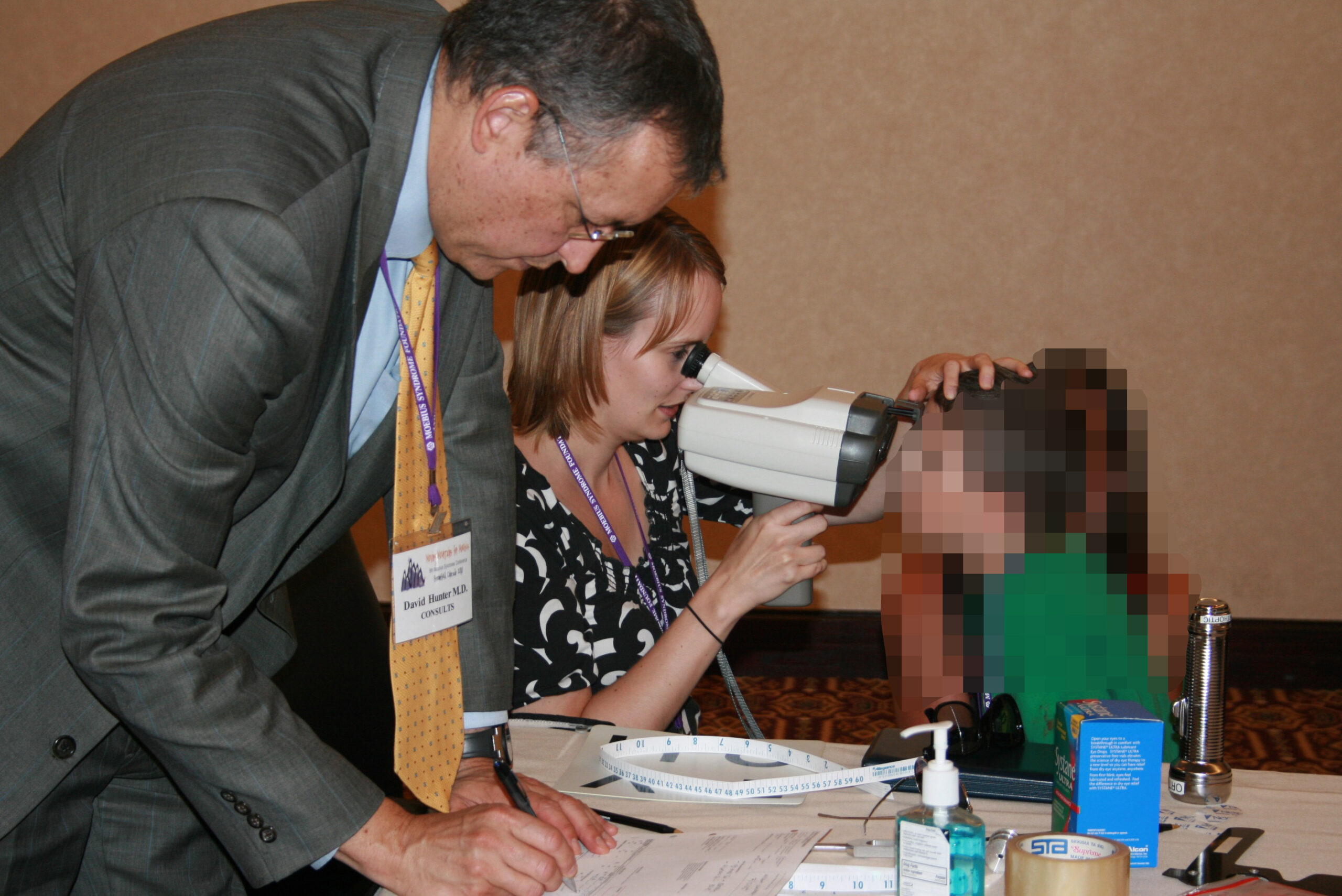
Dr. David Hunter and Sarah Mackinnon (orthoptist) evaluating a patient at the Moebius Syndrome Foundation (MSF) Annual Meeting.
Attending the Ocular CCDD Clinic
Elizabeth Engle MD (Neurologist), David Hunter MD, PhD (Ophthalmologist-in-Chief), Sarah MacKinnon MSc, OC(C), CO, COMT (Chief Orthoptist) and Brenda Barry MS CGC (Genetic Counselor) hold a combined outpatient clinic in which they evaluate individuals of all ages who have, or may have, an ocular CCDD.
CCDD clinic patients undergo ophthalmological and neurological consultations. Diagnosis, etiologies, prognosis, treatment options, genetic testing, and research enrollment options may be discussed at the clinic visit. Drs. Hunter and Engle will both be present to answer questions and address concerns.
Appointments generally last 3 to 4 hours and the clinic is typically held the first Monday afternoon of the month in the Ophthalmology Department, Fegan 4, Boston Children’s Hospital main campus, 300 Longwood Avenue, Boston, MA.
Screening
We request information in advance of CCDD clinic visits. This information allows us to be certain that the patient is seen by the correct specialists and allows us to review the patient’s history in advance. If you or a family member or patient wishes to be screened for CCDD clinic, please download and fill out the four forms by clicking below. Once completed please return them to us via email to englegc.research@childrens.harvard.edu or by regular mail.
Please contact englegc.research@childrens.harvard.edu for more information about the CCDD clinic and/or to schedule an appointment. If an appointment is appropriate, you will be connected with administrative staff at the hospital to obtain a medical records number and to receive help regarding any billing and insurance questions.
Frequently Asked Questions (FAQs)
What is strabismus?
Strabismus refers to eyes that are misaligned. This misalignment, or inability of the eyes to coordinate movements, may be present from infancy or develop over time and is thought to affect 1-5% of the general population. We are still learning about strabismus, but we know it can be caused by problems with the eye muscles, the nerves that control these muscles, or problems with how the brain processes visual information. The Engle Lab studies familial strabismus of all types and complex strabismus syndromes that are present from birth or infancy.
What is a CCDD?
Congenital Cranial Dysinnervation Disorders (CCDDs) are neurological conditions affecting one or more of the cranial motor nerves. The cranial motor nerves develop from the brain and connect to muscles and tissues to control functions such as movement of the eyes and eyelids, facial expression, blinking, chewing, and swallowing. CCDDs that impact eye movements, and often cause strabismus, are called ocular CCDDs. Ocular CCDDs include CFEOM (congenital fibrosis of extraocular muscles), Duane syndrome, 3rd, 4th, and 6th nerve palsy, Moebius syndrome, ocular synkinesis syndromes and horizontal gaze palsy with progressive scoliosis (HGPPS). Other CCDDs most prominently affect facial movements, resulting in congenital facial weakness.
What genes are studied by the Engle Lab?
The Engle Lab has identified, characterized, and continues to study multiple genetic disorders that alter the development and function of the nerves and muscles critical for typical eye and facial movements. Some of the molecules and pathways we study are related to mutations in the following genes: KIF21A, TUBB3, TUBB2B, and PHOX2A that cause CFEOM; CHN1, MAFB, HOXA1, SALL4, and ROBO3 that cause Duane syndrome / horizontal gaze palsy; and HOXB1 that causes congenital facial weakness.
The Engle Lab is also focused on identifying new, previously uncharacterized genetic causes of CCDDs. For this reason, our set-up and methods differ from those of commercial testing labs that exist to precisely test known genes. If your health care provider feels that a known genetic condition may explain the symptoms you or a family member are experiencing, genetic testing for that condition may now be commercially available because of past research successes. If so, it may be more efficient to directly pursue that testing in a clinical lab rather than enrolling in a research study that has an uncertain timeline for results and the need for repeated sampling and confirmation testing.
Is there a cost to participants?
There is no cost to participate and travel is not required. There is also no compensation for participation in our study.
What are the goals of the study?
We use DNA from participants to identify and study new genetic causes of CCDDs and impaired eye and facial movements. The clinical information helps us to understand what a gene does normally and what can result if it is mutated and thus not working normally. From a clinical standpoint, by understanding the genetic cause of a given CCDD we hope to improve diagnosis and treatment, and to provide accurate genetic counseling.
What is the enrollment process?
We welcome contact by email, phone, or postal mail from patients, parents, and healthcare providers to determine if someone is eligible for the study. Once we receive an inquiry and screening forms, research staff contact you to discuss enrollment or request additional information, review the consent form, and guide the sampling process.
What type of medical information is helpful?
We benefit from collecting details through interviews and forms completed by patients themselves or their health care providers, as well as through receipt of primary medical records. Typically, of greatest help are records related to ophthalmology, neurology, and genetics, including exam and surgical notes, testing results, and copies of brain/eye imaging (especially MRI scans). We can also provide a link to an online web app, StrabisPIX, developed by our colleagues in Ophthalmology at Boston Children’s Hospital to capture photographs of eye positions.
What types of samples are needed for participation?
We generally start by obtaining DNA using a specialized saliva collection kit that we provide to families. Sometimes we may ask for a blood draw if collection of DNA using saliva is not successful or if we have a specific need for whole blood. We may also obtain already stored DNA and/or tissue that may have been collected through a doctor’s office and is no longer needed. Most commonly, only one sample is required. However, if the DNA is of poor quality, is used up over the course of the research, or we have a specialized project, we may reach out to enrolled participants to request additional samples, if they have provided consent for us to recontact them.
Who can participate?
We need to enroll and obtain DNA from at least one family member diagnosed with a condition we study. If only one person in the family has the condition, we would likely request that the person’s parents and siblings also enroll. If more than one person in the family has the condition, we would likely ask if all affected individuals and their immediate family members would enroll. This allows us to compare changes we find in the DNA of the persons with the diagnosis to the DNA of unaffected family members to best interpret genetic findings. However, we acknowledge that enrolling additional biological relatives is not always feasible. Importantly, we must consent each participant and receive their sample.
What forms or information are required?
We ask to receive back a general intake and 3 initial forms from people interested in the study. Other materials, including the consent and submission form, are provided by email and/or with the enrollment kit. These forms may be downloaded by clicking below. They can be completed and returned by email or regular mail.
- Intake Form
- Clinical History Form
- Family History Form
- Medical Records Release Form
- Example Consent (this is an example and should not be used for enrollment)
- Sample Submission Form
- Participant Questionnaire (PQ)
Are study participation and results confidential?
Our lab collects DNA samples and medical information from persons and families with features indicating they have or may have a CCDD or familial strabismus. The information is kept confidential. Identifiable details are not shared with anyone outside of the Engle Lab without the specific permission of participants. No results are placed in the medical record of participating individuals or their families prior to clinical confirmation. We consent people to make sure everyone understands important details of the study and how we protect participant data.
Can participants obtain results?
Analyses will be done in our research laboratory and so are considered investigational. This means that we cannot directly release results to a participant. However, participants can call or email us at any time to ask questions or to check in on the overall progress of the program. In addition, participants are given choices on the consent form to indicate if s/he wishes to be informed about the availability of results. If we identify findings for a specific participant who has noted s/he wished to be informed of significant results, we would attempt to contact the participant and/or a designated medical provider (based on information obtained during enrollment or as updated by the participant). We would work with them to have our research findings confirmed by an authorized clinical laboratory which would then issue a report that would become part of the medical record and provide details about the results. Our research findings are also shared through scientific publications. The results in such publications do not include any information that would permit identification of participants. We can provide copies of these publications on request.
What are possible benefits of participation?
Each and every participant contributes to the improved understanding of the CCDDs. Some participants may also learn about the genetic basis of their condition.
How can I tell other people about this study?
You may tell people about this website and share all of our contact information with anyone you wish. You may also download and share these pamphlets about the research on cranial nerves and on facial weakness and Moebius syndrome.




